Leaders in Pediatric Extracorporeal Membrane Oxygenation Therapy (ECMO) and More
In 1988, the Pediatric Intensive Care Unit at Nicklaus Children's Hospital broke new medical ground, becoming the first pediatric center in Florida to offer extracorporeal membrane oxygenation (ECMO) therapy as a life-saving intervention for the region's most critically ill infants and children. Since those pioneering beginnings, the hospital is proud to have given new life to hundreds of children, while maintaining one of the best outcome records in the field.
The program is honored to be a recipient of a Gold Level ELSO Award for Excellence in Life Support from the Extracorporeal Life Support Organization (ELSO).
The ECMO service at Nicklaus Children’s served as the foundation for what is now the Extracorporeal Life Support Program, which includes ECMO, continuous renal replacement therapy and pediatric therapeutic apheresis.
Services are provided through the Pediatric Intensive Care Unit and Cardiac Intensive Care Unit at Nicklaus Children's Hospital.
What is Extracorporeal Membrane Oxygenation (ECMO) for Children?
ECMO is a procedure in which blood is circulated outside the body to provide life-saving support. "Extracorporeal" means "outside the body." During ECMO, the patient's blood is routed through a pump that acts as the heart, circulating blood from patient to the machine. From the pump, the blood then passes through an oxygenator, an artificial lung, which adds oxygen, removes carbon dioxide, and returns the oxygenated blood to the patient.
By replicating the natural functions of the heart and lungs, ECMO provides critical support, enabling a child or newborn’s organs to rest and heal. ECMO is implemented when all other conventional treatments have failed. It is often used for those experiencing respiratory or cardiac failure due to birth defects, trauma, or severe infections. While on ECMO, the child is cared for by a highly skilled, interdisciplinary team, receiving continuous, round-the-clock monitoring and support from the ECMO machine, often referred to as a "heart-lung machine."
During extracorporeal membrane oxygenation treatment, the child's heart continues to beat, but its work is made easier because the ECMO machine does much of the pumping. The length of time a child remains on therapy depends on the diagnosis and the child's individual response.
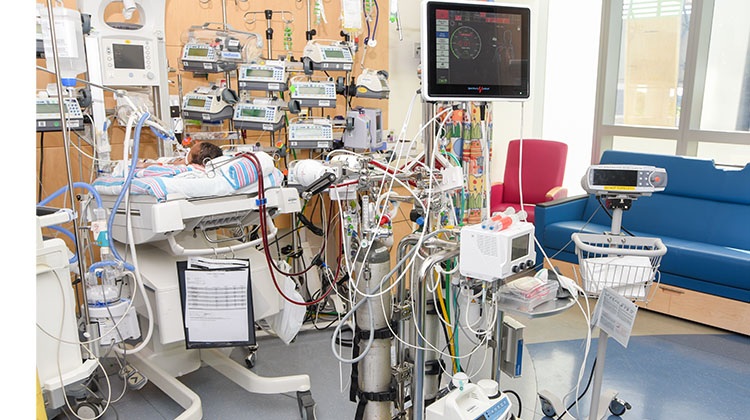
Candidates for ECMO
ECMO is used to help babies with a wide variety of medical challenges, including:
Pediatric ECMO indications following repair
- Acute respiratory distress syndrome (ARDS)
- Sepsis
- Severe pneumonia
- Trauma causing lung injury
- Aspiration pneumonia
- Asthma
Cardiac Failure
Different Types of ECMO
-
Venous Arterial (VA) ECMO — In VA ECMO, a catheter is placed in the vein on the right side of the neck which removes un-oxygenated blood from the body. A second catheter is placed in the artery on the right side of the neck which returns oxygenated blood from the ECMO circuit to the body.
-
Venous Venous (VV) ECMO — In VV ECMO, in a single or double catheter approach blood is removed and returned using vein. VV ECMO only provides support for the lungs, whereas VA provides support to the heart and the lung.
ECMO blood flow is maintained at a sufficient rate to adequately perfuse the patient and allow "rest" of the heart and lungs.
ECMO is also maintained at a level such that adequate oxygen delivery is achieved for patient needs. These oxygenation needs may change from time to time, depending on the patient's condition.
Weaning Patients from ECMO
Initially, when the patient is placed on ECMO, the pump flow is kept high to enable the heart and lungs to rest. As heart and lung functions begin to improve, the ECMO flow will be reduced so that the patient’s heart and lungs are able to do more of the work.
The function of the heart and lungs are measured by blood samples, chest X-rays, chest movement and echocardiogram. Once the patient's condition has improved, the ECMO flow is decreased and the patient is transitioned off of the pump for several hours.
If during this time, the patient remains stable, ECMO can be discontinued. Once ECMO is discontinued, the catheters will be removed from the neck and the vessels will be repaired.
Potential ECMO Complications
Complications that can be associated with ECMO include:
- Internal bleeding may result since the blood must be kept from clotting. A medication that is used to prevent blood clotting can lead to bleeding problems.
- Whenever a tube is inserted in a blood vessel, there is an increased risk of infection, surgical complications and stroke, which may occur since the procedure involves tying off two major blood vessels, typically the carotid and the internal jugular vein.
- Blood clots are another complication of ECMO that can occur even though the circuit is monitored visually for any signs of clots or air.
- Transfusion-related infections may occur due to the fact that patients will be receiving blood and blood products transfusions.
- Technical failure or malfunction can occur just as any piece of equipment can.